cTAP captures the recognized and well-documented benefits that can be achieved by organizing as a consortium to solve big challenges while at the same time avoiding the pitfalls the consortium model can present.
cTAP’s scientific operating model ensures an objective process that is impact-focused and nimble. The consortium is governed by a Joint Steering Committee that prioritizes goals based on the most critical needs for drug development and evaluation. Analytic results, and not raw data, are shared within the consortium while findings are validated across data sources.
cTAP delivers real value to therapy developers and academic collaborators by keeping the focus on the goals that matter most.
The Duchenne community has been prescient in developing a wealth of natural history. However, prior to cTAP these data were curated and analyzed within silos because the field lacked effective mechanisms for bringing datasets together across clinical networks.
cTAP has lowered the barrier for clinical centers, clinical registries, and drug company sponsors of clinical trials to share de-identified individual patient clinical data, and has markedly accelerated the cycle-time between idea, analysis, and results by providing:
- a secure data repository and data harmonization for all cTAP collaborators;
- a collaborative analytics platform to mine de-identified patient data in real time; and,
- a simple and equitable means of disseminating pre-publication results and findings to multiple drug developers simultaneously.
From Months/Years to HOURS/DAYS
Read the latest
News, press and peer-reviewed publications by cTAP collaborators
In partnership with the Analysis Group, cTAP brings advanced data science to learn from patient data. Studies are conducted in collaboration with academic clinical experts and results are shared with cTAP members.
cTAP’s access to a large and growing database of patient outcomes in Duchenne enables us to:
- discover in one database source, replicate in others;
- conduct Individual patient level analyses as well as analyses on cohorts;
- analyze longitudinal trajectories as well as cross-sectional data;
- compare analytic results across geographies, clinical practice versus clinical studies, natural history versus placebo; and,
- develop consensus models from aggregated, pooled data.
Sharing the knowledge created within the consortium with the scientific community is a critical principle of cTAP. cTAP disseminates new scientific findings promptly at key conferences and publishes insights in peer-reviewed journals. Academic experts regularly include cTAP studies and scientific insights in invited plenary presentations. Engagement with regulators serves to prime the pump on translation of findings to trial design and interpretation. cTAP also participates in patient organization conferences and provides lay updates on progress as appropriate.
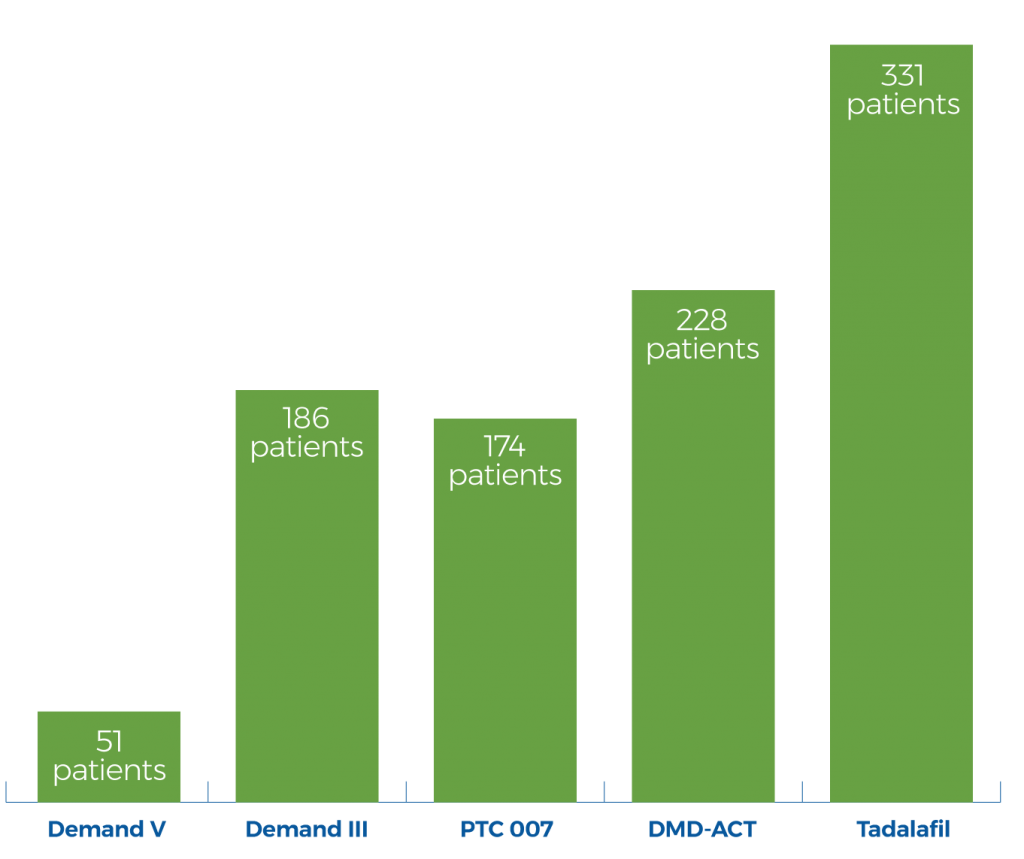
Pivotal Trials Have FAILED to Meet Their Primary Endpoint