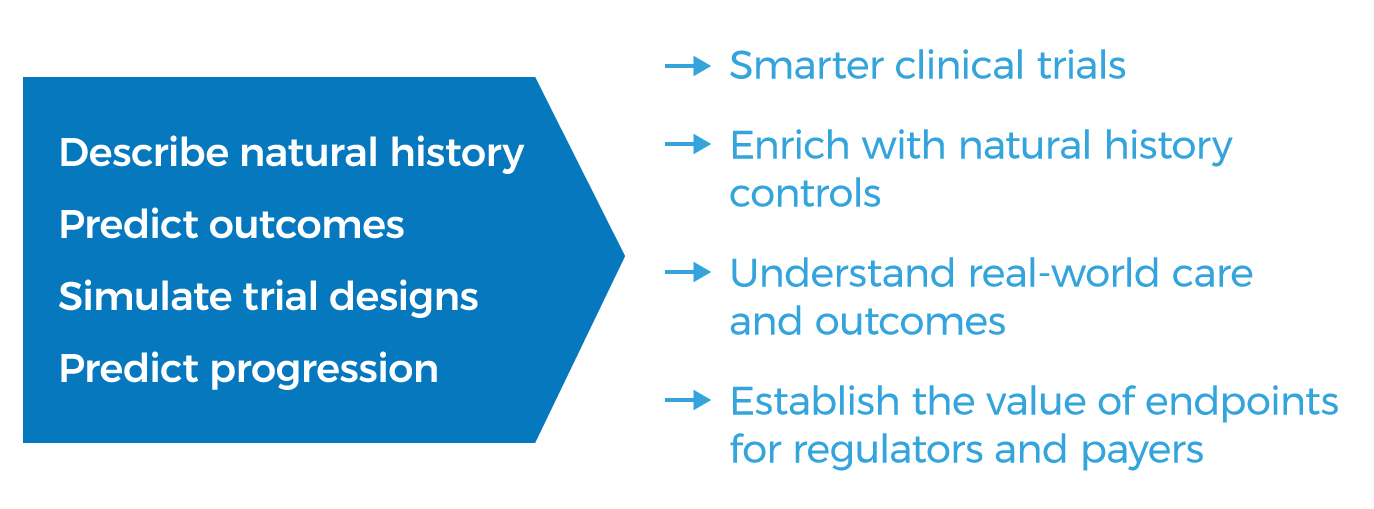
Smarter clinical trials are trials designed to leverage natural history and real-world data to yield clear, unambiguous understanding of the efficacy of a new therapy.
The high rate of pivotal trial failures in Duchenne leaves most of the 300,000 boys and young men with Duchenne muscular dystrophy without the right treatment. Each patient with Duchenne has his own distinctive longitudinal trajectory of disease progression. Some patients progress rapidly, while others may only reach the same clinical milestones a decade or more later.
The core challenge in designing and analyzing clinical trial and post-marketing data in Duchenne is how to take into account these different rates of disease progression to discern whether a patient is doing well because of a drug or because his disease is slowly progressing and he was going to do well anyway.
To understand and account for heterogeneity in longitudinal progression of disease by:
- Engaging regulators, clinical experts, drug developers and patient groups in collaborative learning from patient data
- Creating tools and insights for drug development and evaluation
- Sharing broadly with the entire Duchenne community
- Delivering near-term impact for clinical trials
Smarter trials, therapies to patients sooner
cTAP has established collaborations to access patient level clinical data from large clinical centers treating patients with Duchenne, clinical registries that curate data across multiple centers, and drug companies that have conducted clinical trials in patients with Duchenne.
Read the latest
News, press and peer-reviewed publications by cTAP collaborators
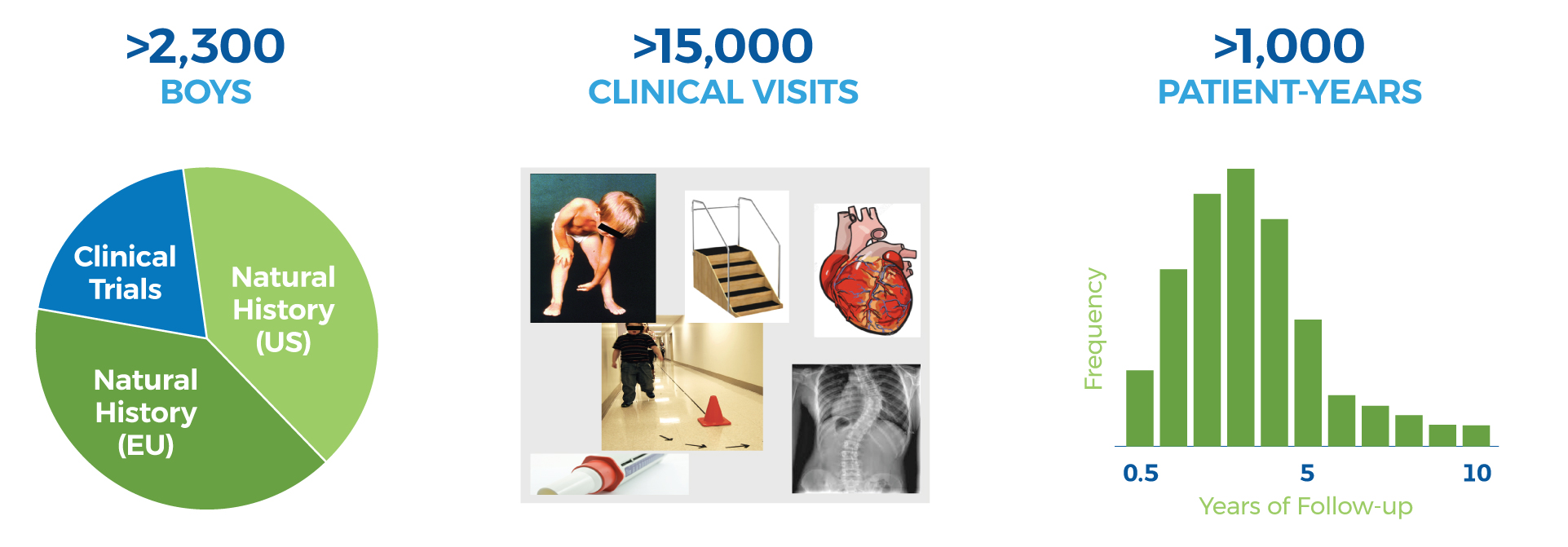
This large and growing database of patient outcomes in Duchenne enables cTAP to:
- conduct individual patient level and cohort analyses;
- analyze longitudinal trajectories as well as cross-sectional data;
- compare analytic results across geographies, and data from the real world and natural history versus clinical studies; and,
- develop consensus models from aggregated, pooled data.
cTAP has:
- characterized and quantified the heterogeneity in longitudinal disease progression that historically has undermined clinical trials in Duchenne;
- identified prognostic factors to more than double prognostic accuracy in outcomes over the period of a clinical trial;
- established the consistency of progression of outcomes between natural history and placebo arms; and,
- defined quantitative relationships between near term changes in outcomes versus time to longer term clinical milestones.
cTAP Pipeline
| PROGRAM | PATIENT DATA | ANALYTICS | PUBLICATION | REGULATORS, PAYORS/HTAs* | ADOPTION* |
|---|
| CHARACTERIZATION |
| PREDICTION |
| SIMULATION |
| EXTERNAL CONTROL FOUNDATIONS |
*With primary target group – academic, regulators, payors/health authorities – dependent on program status by leading outcome measure
Less than two years after it was founded, cTAP had published two significant and influential peer-reviewed articles, and a third is now in press. Each year, cTAP collaborators have presented at least five abstracts describing new findings at the premier conferences in neuromuscular and pediatric rare diseases, including the World Muscle Society. Read below for examples of research questions addressed or work underway.
Characterizing heterogeneity of disease progression
Quantitative evidence of underlying structure to differences in disease progression – Clustering of longitudinal trajectories of natural history halves variance.
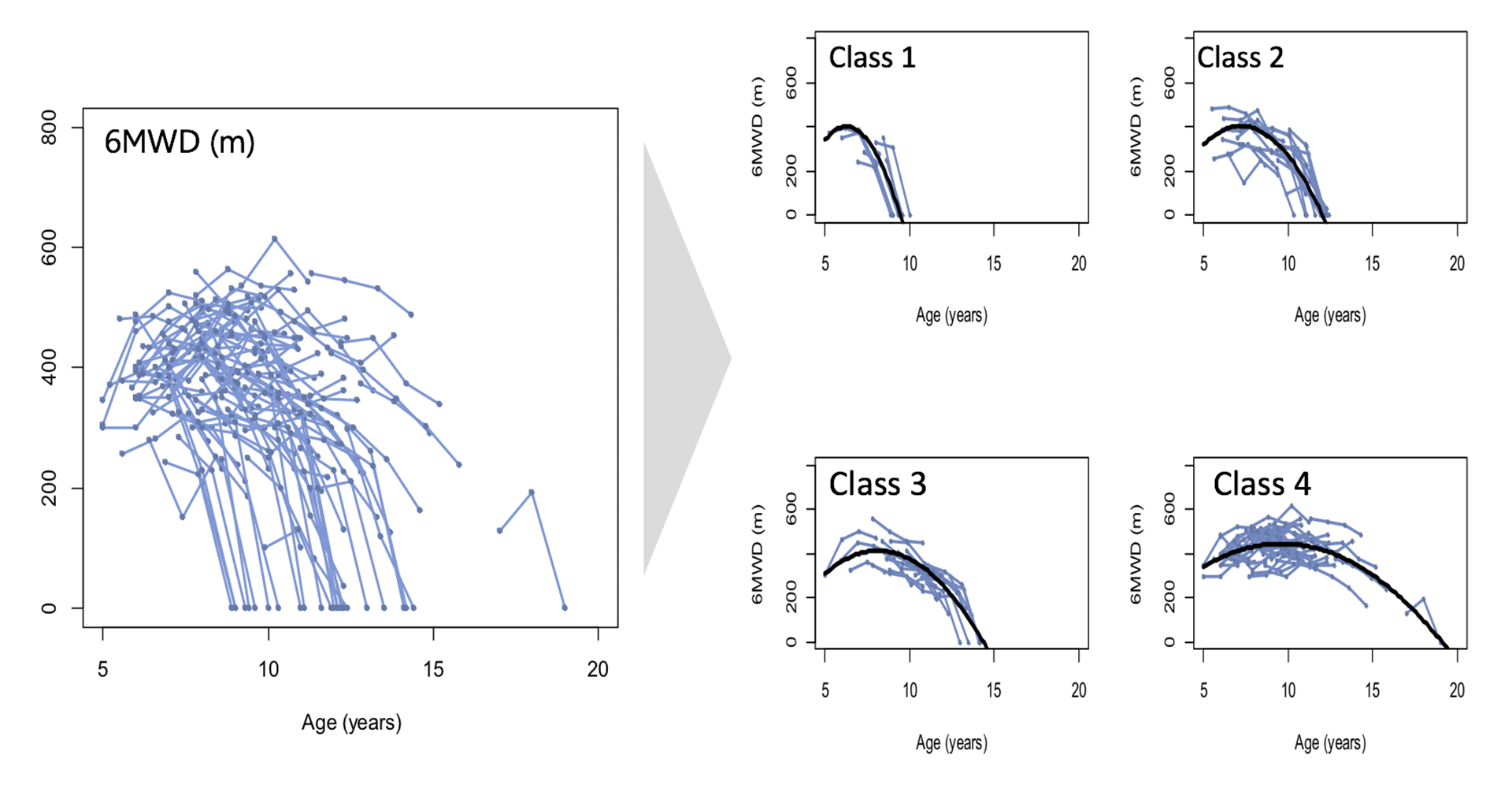
Mercuri EM, Signorovitch JE, Swallow E, Song J, Ward SJ for DMD Italian Group and CollaborativeTrajectory Analysis Project (cTAP) (2016)..Categorizing natural history trajectories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscular Disorders; (26): 576-583
Translation to drug development
cTAP research is highly applied. The research plan embraces studies addressing key questions posed by regulators, answers to which can be deployed broadly by all cTAP members.
Prognostic factors identified and prognostic models built – more than doubles prognostic power, marked reduction in ‘n’ to power a clinical trial.
Total sample sizes required to detect a 30 m change in 6MWD
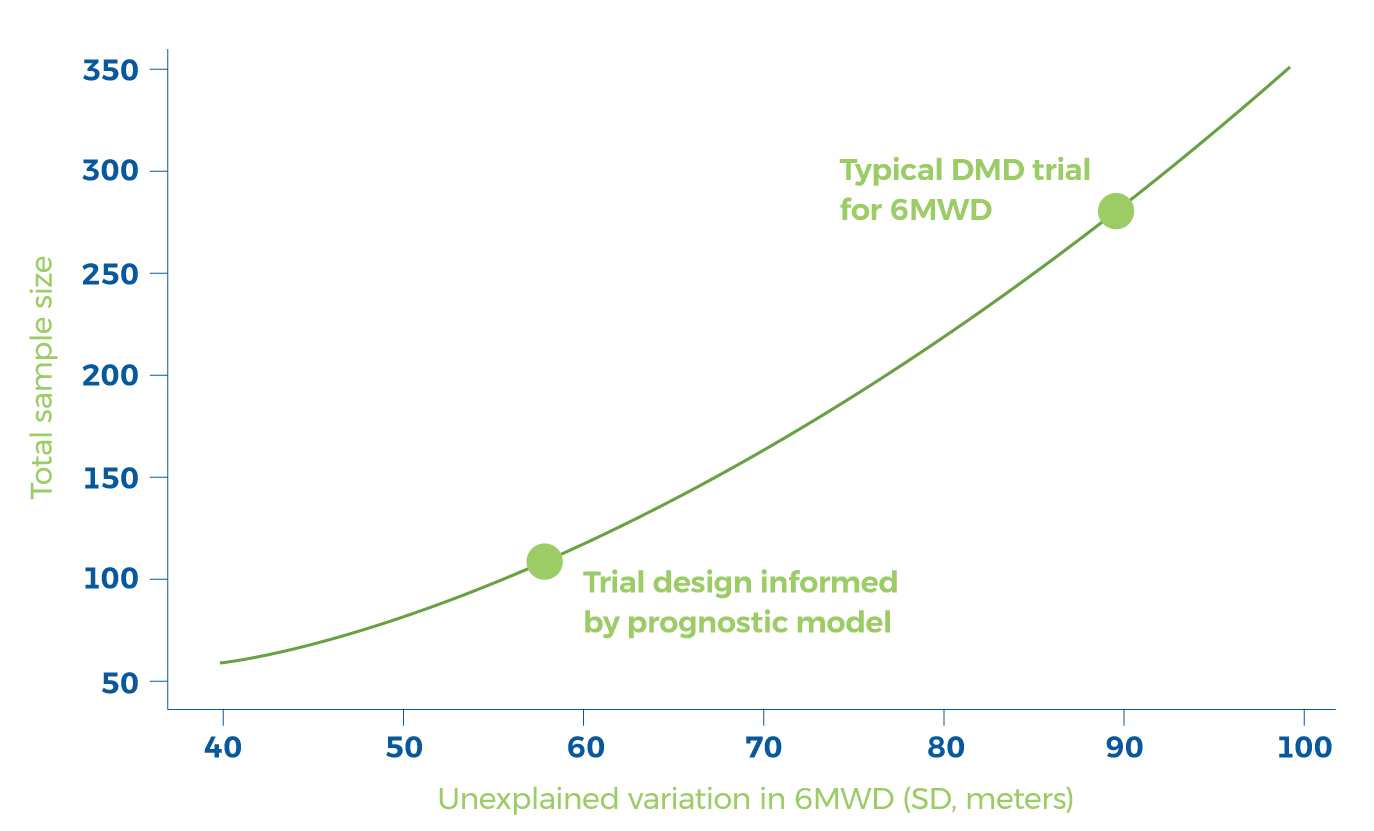
Goemans N, vanden Hauwe M, Signorovitch J, Swallow E, Song J, CollaborativeTrajectory Analysis Project (cTAP) (2016). Individualized Prediction of Changes in 6-MinuteWalk Distance for Patients with Duchenne. Muscular Dystrophy. PLoS ONE 11(10): e0164684
Tools to pressure test clinical trial design
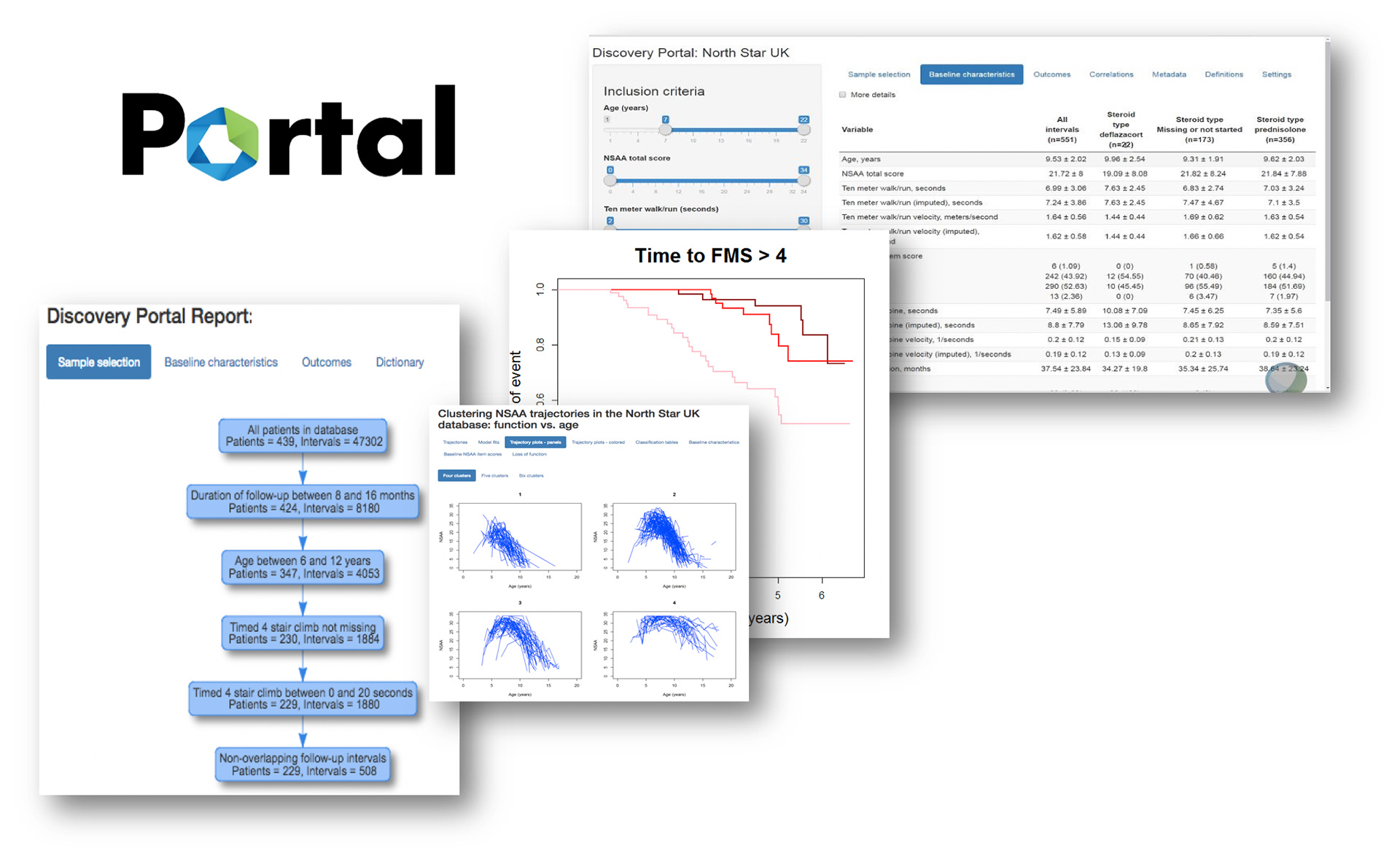
Harmonized data
Dynamic analytics
Rapidly testing hypotheses
Timely replication and extension



































